Protocol
Accuracy of established manual versus new semi-automated liver volumetry software in patients undergoing liver surgery and living donor liver transplantation
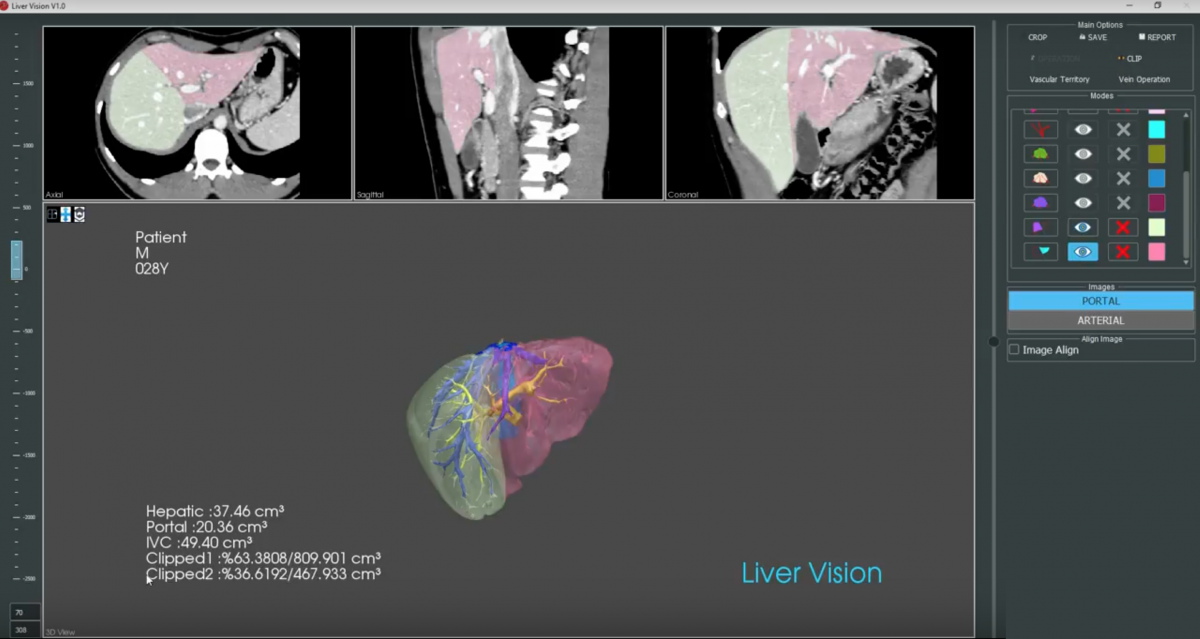
Background
Liver volumetry has been widely used in liver surgery and living donor liver transplantation to estimate the future liver remnant or the required liver volume for the recipient, respectively.
Liver volumetry is typically performed using CT imaging with specially designed software. Such manual measurements are time consuming and there is an ongoing debate whether the they reflect the actual liver volume when performed by radiologists or surgeons as well as according to the different software currently available in the market.
Objectives
The aim of this study is to assess the accuracy and functionality of a new semi-automated liver volumetry software (LiverVision® software) in predicting the clean future liver volume in patients undergoing liver surgery.
Study design
Stage 1 - Validation
Prospective validation of the new semi-automated volumetry software (LiverVision®) will be conducted in living liver donors undergoing partial hepatectomy. The reference standard will be the true weight of the donor grafts immediately after resection and prior to transplantation. The volumetric measurements of the new semi-automated software will be compared with those of existing, established manual measurements typically performed by radiologists and liver surgeons (PACS). The interclass correlation coefficient among the different software as well as among radiologists and surgeons will be further assessed.
Registration
This protocol is registered at ClinicalTrials.gov - NCT03766633
Participants
Patients
For the first stage of this project (i.e. the Validation Phase), preoperative CT scans from 108 cases will be collected and assessed. The sample size calculation revealed at least 63 living liver donor consecutive cases (assuming an r2 of 0.90 and 95% Confidence Interval Half-Width 2w of 0.10, two-sided alpha error of 0.05 and power 0.80) will be collected and assessed. To facilitate several subgroup analyses, adjust for dropouts due to poor quality images, and increase precision, the investigators decided to include all available living donor CT cases among the three collaborating institutions. This lead to a total sample size of 108 cases (i.e. 70% oversampling).
Doctors / Raters
Six radiologists comprising of three consultants and three juniors, as well as six surgeons, comprising of three consultant liver surgeons and three HPB fellows will independently perform volumetric measurements.
Test methods
Index test
The index test will be the new semi-automated liver volumetry software measurements for both study stages mentioned above.
Alternative tests
The alternative tests will be the measurements derived from the established manually measured volumetry software (e.g. Osirix®, MeVis®, or local PACS).
Reference standard
For the validation part of this study, the reference standard will be the weight of the liver graft.
Information available to the doctors
The 12 doctors (raters) participating in this study (6 radiologists and 6 surgeons) will be provided with fully anonymised basic demographics, disease characteristics and intended surgical procedure (including liver segments). None of the participants will be aware of the liver graft weight (reference test) and the standardized future liver remnant. Similarly, the raters will not be provided with any patient outcome data.
Click here to download the Case Report Form (list of parameters for data capture) in PDF format.
Analysis
Methods for estimating and comparing measures of accuracy
The intraclass correlation coefficient will be estimated using the data derived from the different raters, the different software, the actual weight of the liver graft or the standardised future liver remnant. The Cronbach’s alpha will be used to assess the internal consistency and the Bland Altman analysis to determine the intervals of agreements. Subgroup analyses will be conducted comparing the measurements derived from the different raters and the different software. Descriptive statistics will be performed to characterise the target population and the raters. The different measurements will be also compared using comparison of mean tests.
Variability
The variability of the measurements will be reported as standard deviation, standard error of the mean and confidence intervals, where applicable.
Results and Discussion
The results will provide clinically meaningful information on whether there are any differences in the liver volumetry measurements performed by radiologists or surgeons as well as whether the new semi-automated software is more useful to existing ones. More accurate measurements translate into safer living donor liver transplantation and liver surgery.
The LiverVision.org Team
